J. Phys. Chem. A 2018, 122, 3, 832-842
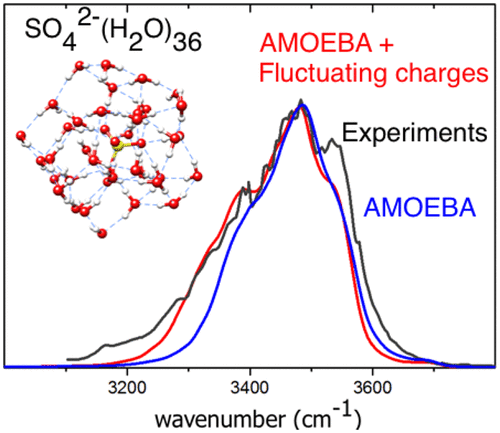
Hydrated ions are ubiquitous in environmental and biological media. Understanding the perturbation exerted by an ion on the water hydrogen bond network is possible in the nanodrop regime by recording vibrational spectra in the O–H bond stretching region. This has been achieved experimentally in recent years by forming gaseous ions containing tens to hundreds of water molecules and recording their infrared photodissociation spectra. In this paper, we demonstrate the capabilities of a modeling strategy based on an extension of the AMOEBA polarizable force field to implement water atomic charge fluctuations along with those of intramolecular structure along the dynamics. This supplementary flexibility of nonbonded interactions improves the description of the hydrogen bond network and, therefore, the spectroscopic response. Finite temperature IR spectra are obtained from molecular dynamics simulations by computing the Fourier transform of the dipole moment autocorrelation function. Simulations of 1–2 ns are required for extensive sampling in order to reproduce the experimental spectra. Furthermore, bands are assigned with the driven molecular dynamics approach. This method package is shown to compare successfully with experimental spectra for 11 ions in water drops containing 36–100 water molecules. In particular, band frequency shifts of the free O–H stretching modes at the cluster surface are well reproduced as a function of both ion charge and drop size.