Water confined in hydrophobic microporous materials
When a fluid is confined in a small volume, its properties can be greatly modified. Phase equilibria are especially affected by confinement: modification of the transition temperature, spreading of the transition over a range of temperature,... The case of water is particularly interesting, because the hydrogen bond networks is strongly frustrated when water is confined in nanometric pores. The precise knowledge of these modifications may help to understand some biological phenomena like the folding of proteins, but may also lead to new applications (wastye water treatment,...)
During my post-doc in Orsay (at the LCP) and then in Paris (at the ENSCP), my research work focused on the study of the properties of water confined in hydrophobic porous materials, using molecular simulations. More specifically, the hydrophobic materials were all-silica zeolites. This work has been made in collaboration with Dr Anne Boutin, Prof Isabelle Demachy, and Prof Alain Fuchs in Orsay and Paris, and in collaboration with Dr Joël Patarin, Dr Michel Soulard, and Dr Mickaël Trzpit of the LMPC in Mulhouse.
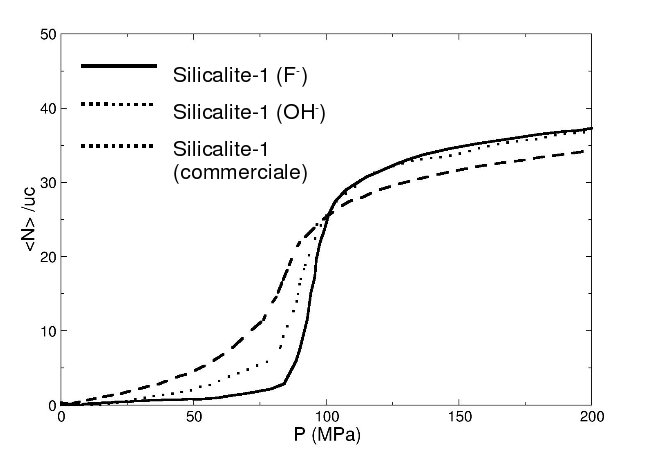
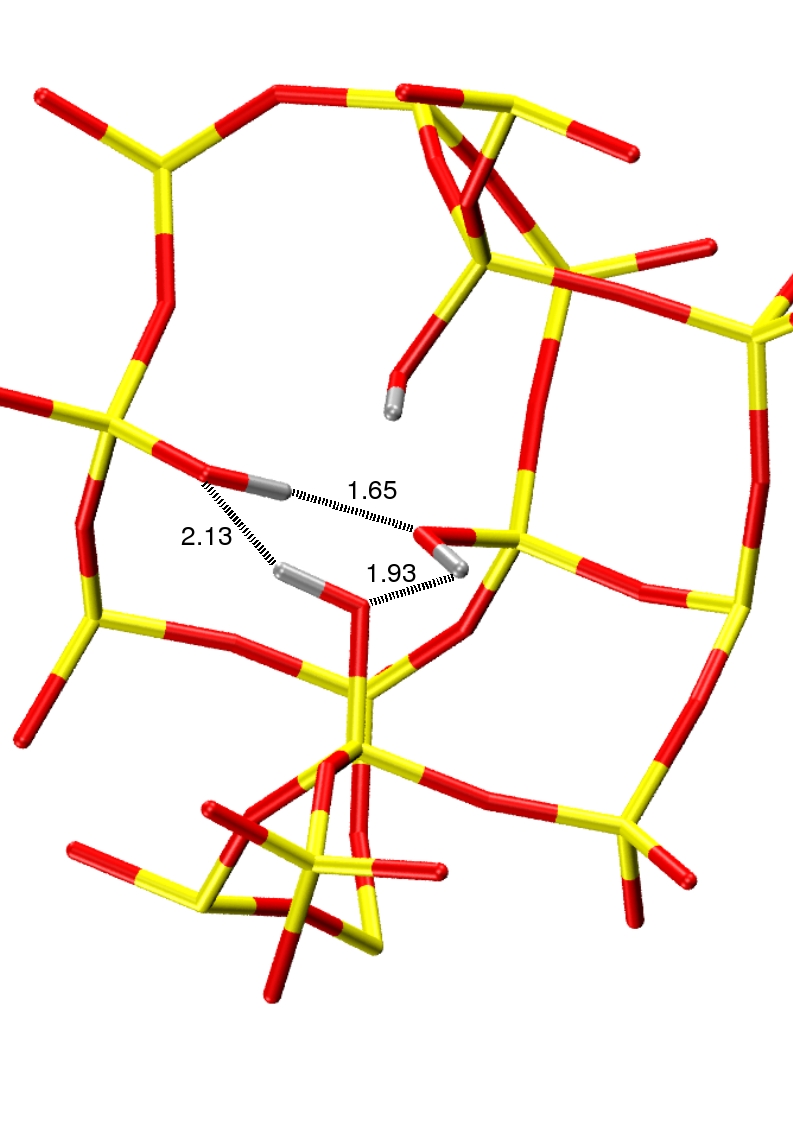
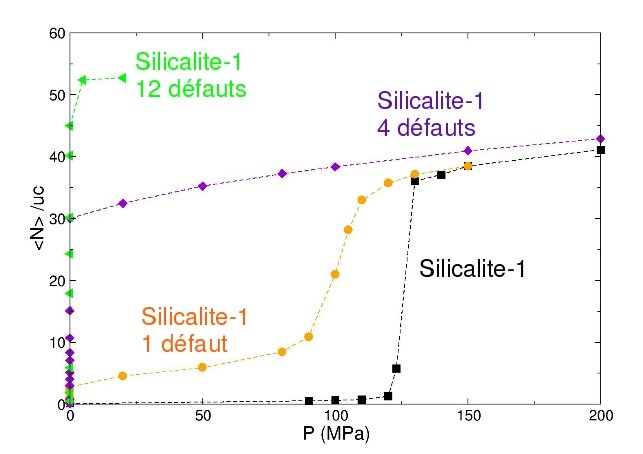
We first studied the influence of the presence of local hydrophilic defects in the zeolite structure on th water intrusion isotherm. Experimentaly it was indeed observed that modifying the protocol of the synthesis of the silicalite-1 leads to modification of the intrusion isotherm. These modications were correlated with the presence of hydrophilic OH-groups inside the structure. We have been able to reproduce these experimental results with the use of Monte Carlo simulations, by introducing silanol nest defects inside the silicalite-1 framework. We have also been able to study the modification of the thermodynamics of the transition with respect to the number or the strength of the local defects.
 |
 |
 |
| Exprimental intrusion isotherm of water in silicalite-1 | Silanol nest |
Simulated intrusion isothermsof water in silicalite-1 with different number of local hydrophilic defects |
We then explored the thermodynamics of the water intrusion process in various hydrophobic zeolite framewoks, in order to rationalize experimental observations. To this end, we have implemented a Wang-Landau algorithm adapted to the Grand-Canonical ensemble in the GIBSS Monte Carlo simulation code developped at the LCP and IFPEN. This allowed us to compute the Grand Potential of the system zeolite/water as a function of the pressure and water loading. We have thus been able to demonstrate that the liquid/vapour phase transition observed in silicalite and zeolite β at ambiant temperature is a first-order transition. On the other hand, this transition in the ferrierite is a continuous transition, with only one stable state at each pressure. We finally have been able to give a full picture of these transitions, in terms of a simplified phase diagrams of water in hydrophobic confined media.
Key words
Monte Carlo, zeolites, adsorption, intrusion, free energy, Wang-Landau algorithm
Publications
- "The effect of local silanol defects on water adsorption in silicalite-1 zeolite. A joint experimental and molecular simulation study". M Trzpit, M Soulard, J Patarin, N Desbiens, F Cailliez , A Boutin, I Demachy, and AH Fuchs, Langmuir, 2007, 23: 10131-39.
- "Does water condense in hydrophobic cavities? A molecular simulation study of hydration in heterogeneous nanopores". F Cailliez , G Stirnemann, A Boutin, I Demachy, AH Fuchs, Journal of Physical Chemistry C, 2008, 112(28): 10435-45.
- "Thermodynamics of water intrusion in nanoporous hydrophobic solids". F Cailliez , M Trzpit, M Soulard, I Demachy, A Boutin, J Patarin, AH Fuchs, Physical Chemistry Chemical Physics, 2008, 10(32): 4817-26.
- "Thermodynamic study of water confinement in hydrophobic zeolites by Monte Carlo simulations". F Cailliez , A Boutin, I Demachy, AH Fuchs, Molecular Simulation, 2009, 35(1-2): 24-30.
- "Water nanodroplets confined in zeolite pores". FX Coudert, F Cailliez , R Vuilleumier, AH Fuchs, A Boutin, Faraday Discussions, 2009, 141: 377-98, disc 443-65.